URIDOCTOR
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- URIDOCTOR
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- urine analyzer, portable analyzer, urine analyser
- Category
- Medical Analyzer
Apply a video call to the Supplier

DFI Co., Ltd.
- Verified Certificate
-
16





| Product name | URIDOCTOR | Certification | - |
|---|---|---|---|
| Category | Medical Analyzer | Ingredients | - |
| Keyword | urine analyzer , portable analyzer , urine analyser | Unit Size | 188.0 * 74.0 * 77.0 mm |
| Brand name | URIDOCTOR | Unit Weigh | 430 g |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 902780 |
Product Information
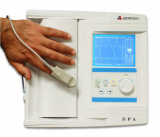
Semi-Automated Single-test Analyzer R-50S
※ Product Features
Light and Portable analyzer
User friendly designed with compact size
Colorful 4.3 inch touch LCD Display
Possibility with 8-battery 1.5 V
Rapid test result in 1 minute
Auto-Calibration function (No calibration strip needed)
Accessible to laboratory information system(LIS) by PC protocol
※ Technical Specification
Throughput : 45 tests/hour (Max 120 tests/hour)
Power : 12V DC/3.33A or Battery 1.5V x 8EA
Memory : 2,000 tests
Net weight : 460 g
Available up to 11 parameters
Optional item : External printer or Bluetooth
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Yun Jung Geon
- Address
- 388-25, gomoro, jillyemyeon,gyeongsangnamdo,korea, Gimhae-si, Gyeongsangnam-do, Korea
- Product Category
- Medical Analyzer,Other Health Care Products,Other Monitoring & Diagnostic Equipment,Other Pharmaceuticals,Veterinary Instrument
- Year Established
- 1996
- No. of Total Employees
- 51-100
- Company introduction
-
DFI Co., Ltd. is a global medical company with the mission to significantly improve the lives of individuals by innovation in meeting consumers' desires all around world. For many years DFI's R&D team has been researching and developing to manufacture diagnostic medical supplies, cooperating with various organizations such as KIST (the Korea Institute of Science & Technology) and famous research professors in diagnostic business circles. Backed by these R&D efforts, our state-of-art production facilities and skilled engineers, made it possible to produce DFI's excellent products with low price & high quality and CYBOW series are first series of our In Vitro Diagnostic products for Urinalysis. Clinical laboratories and medical researchers use our products to analyze urine, blood from patients for the diagnosis of various diseases and other medical complications, or to measure the level of specific substances which may exist in extremely small concentrations in the human body. We look forward to continued growth, the opportunity to explore the ever-expanding and exciting frontiers of diagnostic technology and promise to provide only the best products available to everyone. We thank you for your continued support and trust in our products.
- Main Markets
-
 France
France
 Germany
Germany
 Israel
Israel
 Turkey
Turkey
 U. Kingdom
U. Kingdom
- Factory Information
-
DFI Co., Ltd.
- Main Product



































 South Korea
South Korea





_2.jpg)
