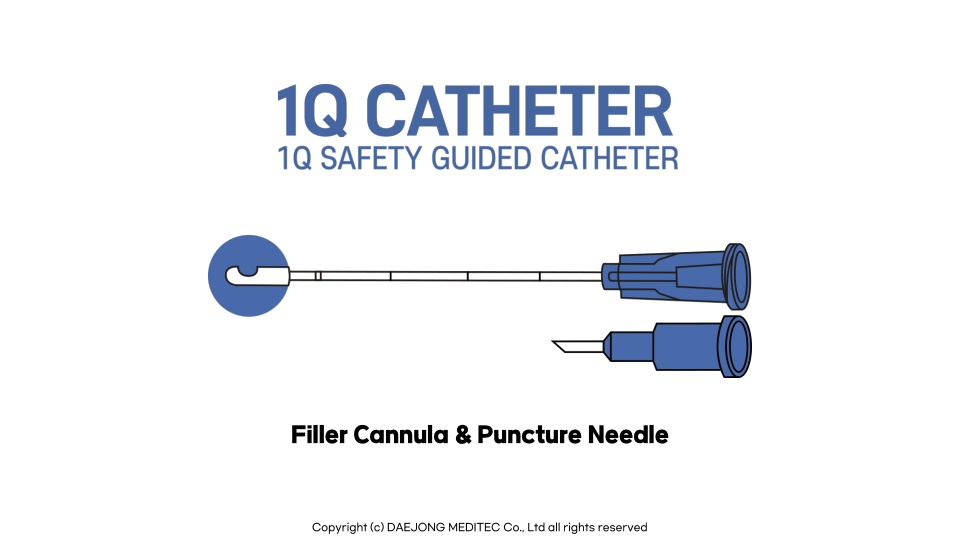
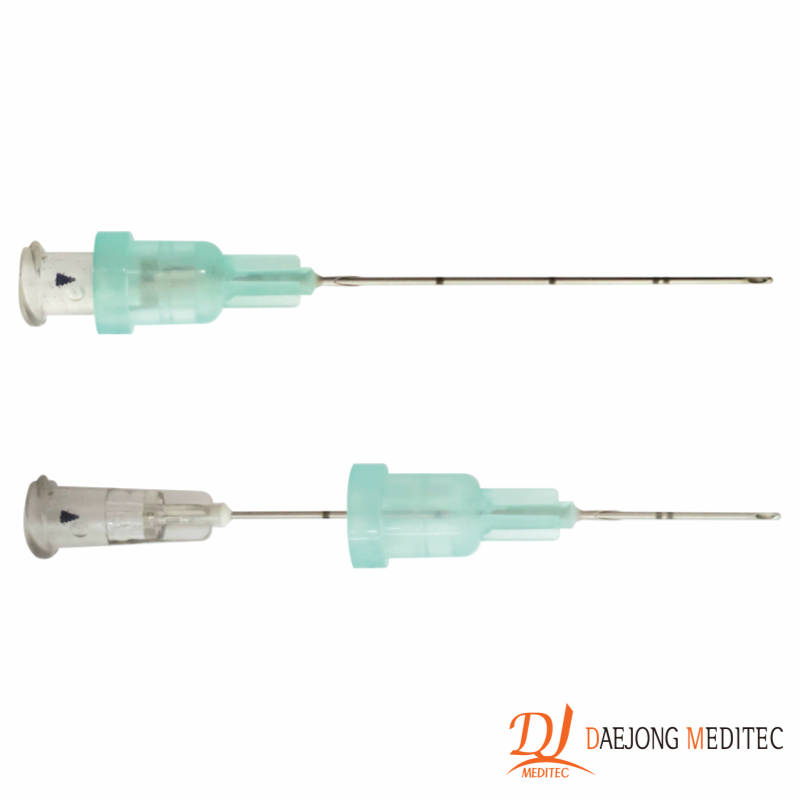
1Q CATHETER
Filler Cannula & Puncture Needle
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others,T/T,Western Union
- Production method
- Available,OBM,ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Other Medical Consumables
Apply a video call to the Supplier

DAEJONG MEDITEC Co., Ltd.
- Recent Visit
- May 09, 2024
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Trading Company
- Verified Certificate
-
4





| Product name | 1Q CATHETER | Certification | - |
|---|---|---|---|
| Category | Other Medical Consumables | Material | - |
| Keyword | cannula , catheter , filler , needle | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 999999 |
| Supply type | Available,OBM,ODM,OEM | HS code | 9018321000 |
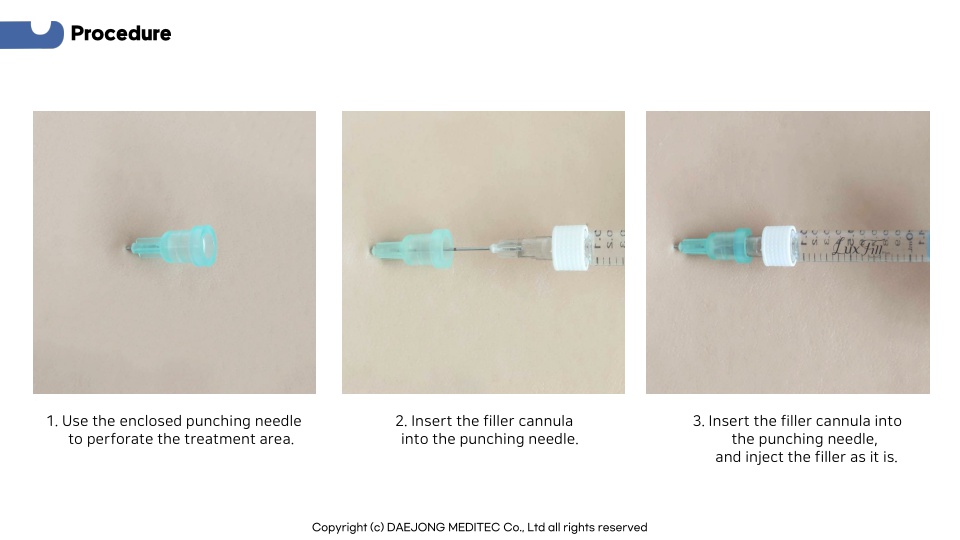
Product Information
1Q CATHETER
Filler Cannula & Puncture Needle
which minimizes skin damage.
of the filler cannula and the inner diameter of the punching needle,
which increases the precision of the procedure.




B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Land Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others,T/T,Western Union | Shipping time | Negotiable |

- President
- LEE SAM GUK
- Address
- 809 57 Achasan ro 17gil, Seongdong-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2015
- No. of Total Employees
- 1-50
- Company introduction
-
What DoesDAEJONG MEDITEC do?
“Based on over 50-year career,
Daejong Meditec constantly responds to the market changes”
We shall manufacture and sell hospital and home medical equipment,
a wide range of beauty care products at home and overseas hospital,
beauty shop, and retailers.
Daejong Meditec after establishing a manufacturing plant in 2007,
does pharmaceutical manufacturing and qualified quality control standards (KGMP), ISO9001, and has a patent pending.
In addition, Daejong Meditec is OEM, ODM certified company as the basis of 50 year experience has been supplied to both domestic and international buyers.
History of DaejongMeditec Co., Ltd
- Signed Exclusive Sales Contract for ‘PCL Barbed Sutures (Lifting Thread Product of ‘ITVP’)’ in Korea, China, Japan, and ASEAN in May 2017.
- Achieved a license of Venture Company Certification in May 2018.
- Established a foreign branch (Daejong Meditec (Thailand)) in Bangkok, Thailand in 2018.
- Awarded “USD 10 million Export Tower” On The 55th Day of Export (R.O.K Presidential Award in December 2018.
- Launched Daejong Meditec’s Filler product ‘LuxFill’ in 2018.
- Got FDA USA approved for the medical device ‘Multi-tap’ in June 2019 ( Registration Number 3015472059).
- Got FDA Thailand approved for medical devices ‘Cannula’, ‘MICRONEEDLES’ in July 2019.
- Certified ISO13485 in September 2019.
- Plan to launch Daejong Meditec’s product ‘Finetox’ in March 2020.
- Main Product
- Attached File



































_2.png)

_2.jpg)

_POWER_COL_TRA_2.jpg)
_2.jpg)
_POWER_BOOSTER__for_Skin_Rejuvenation_2.png)

