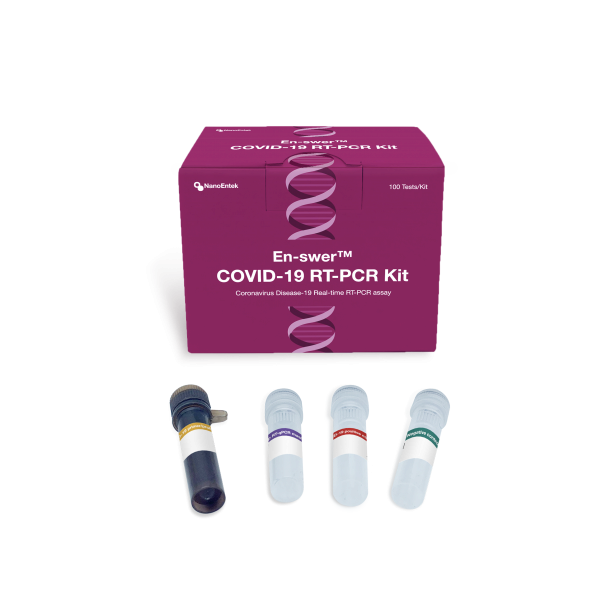
The En-swer COVID-19 RT-PCR Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona virus, real-time pcr machines, rt-pcr, covid-19
- Category
- Medical Devices
Apply a video call to the Supplier
NanoEnTek Inc.
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
16



| Product name | The En-swer COVID-19 RT-PCR Kit | Certification | - |
|---|---|---|---|
| Category | Medical Devices | Ingredients | - |
| Keyword | corona virus , real-time pcr machines , rt-pcr , covid-19 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
1-step Real-Time RT-PCR assay
The En-swer™ COVID-19 RT-PCR Kit detects E, N, and RNaseP genes of SARS-CoV-2 qualitatively through real-time reverse-transcription polymerase chain reaction. It can be used for rapid detection and outbreak control of COVID-19.
KEY FEATURES & BENEFITS
- about 1 hour 30 min | Test time
- 1 tube reaction | Easy to use
- E gene, N gene (Target), RNaseP (IPC) | Detection
- High Sensitivity & Specificity
- Based on WHO & CDC recommended gene
Total Solution for COVID-19
NanoEntek provides a total solution for the detection and surveillance of COVID-19 infection. En-swer™ COVID-19 RT-PCR Kit detects the presence of SARS-CoV-2 virus itself through real-time reverse-transcription polymerase chain reaction. FREND™ COVID-19 IgG/IgM Duo is a point-of-care testing (POCT) which can be used to check whether patients have developed immune responses to SARS-Cov-2 using human serum or plasma.
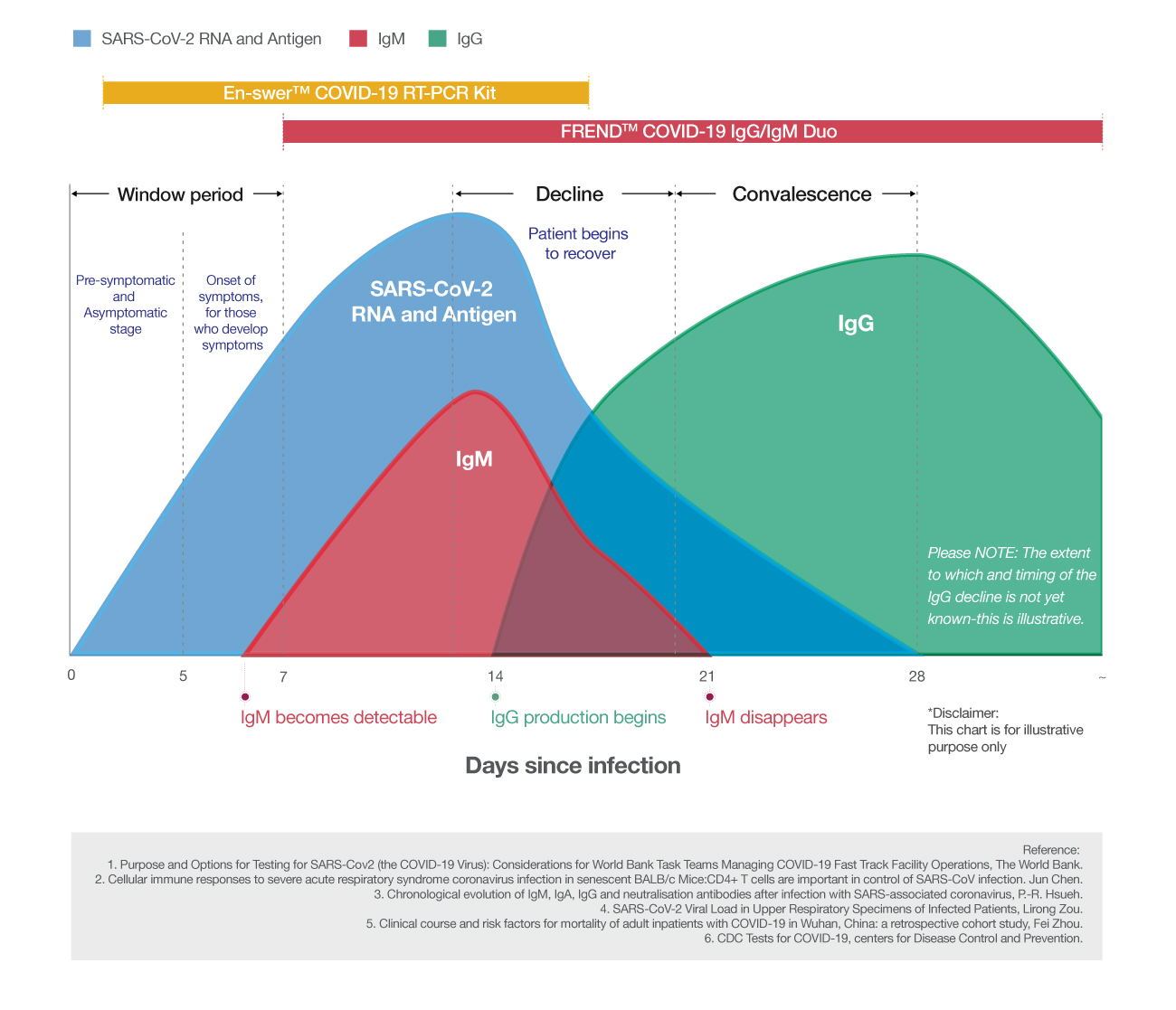
Choose the right COVID-19 test based on your needs.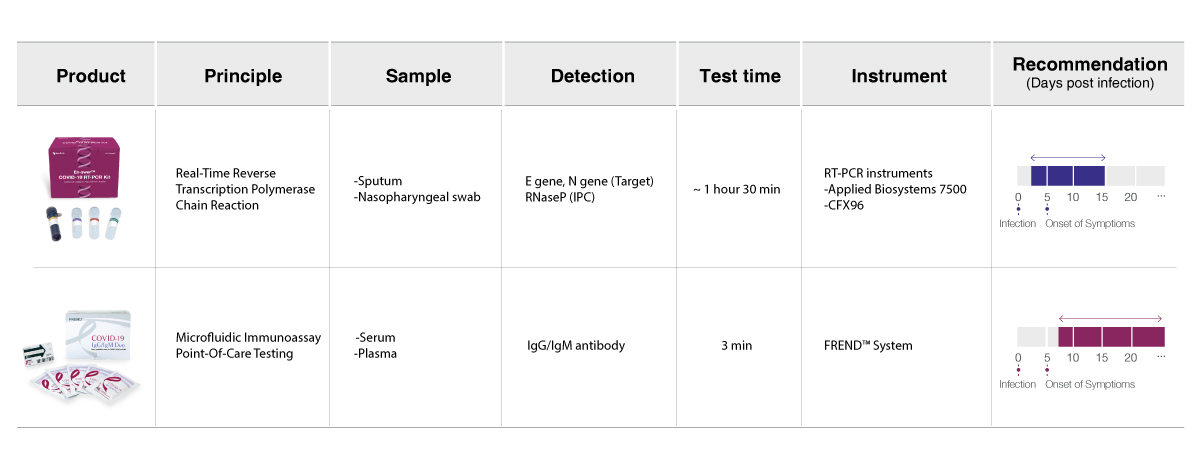
1-step Real-Time RT-PCR assay
En-swer™ COVID-19 RT-PCR Kit
The En-swer™ COVID-19 RT-PCR Kit is an in-vitro diagnostic medical device using 1-step RT-qPCR(real-time reverse-transcription polymerase chain reaction) which is a test method that detects genes(the N gene, the E gene) of the novel coronavirus(COVID-19) through qualitative analysis from sputum, nasopharyngeal swab of patients suspected of being infected with the novel coronavirus(COVID-19).

Instructions for use
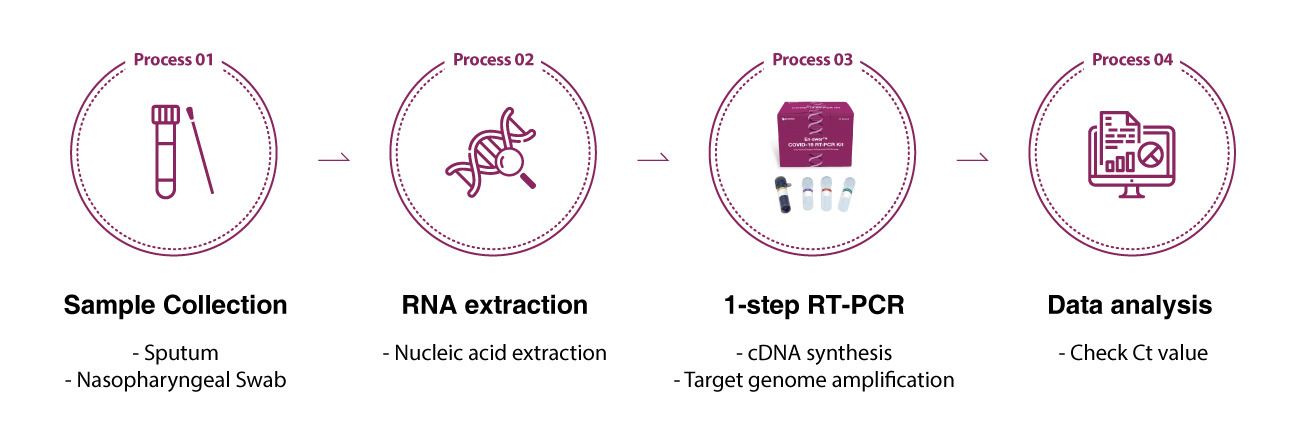
Working Process
The following workflow is the instructions of using En-swer™ COVID-19 RT-PCR Kit.
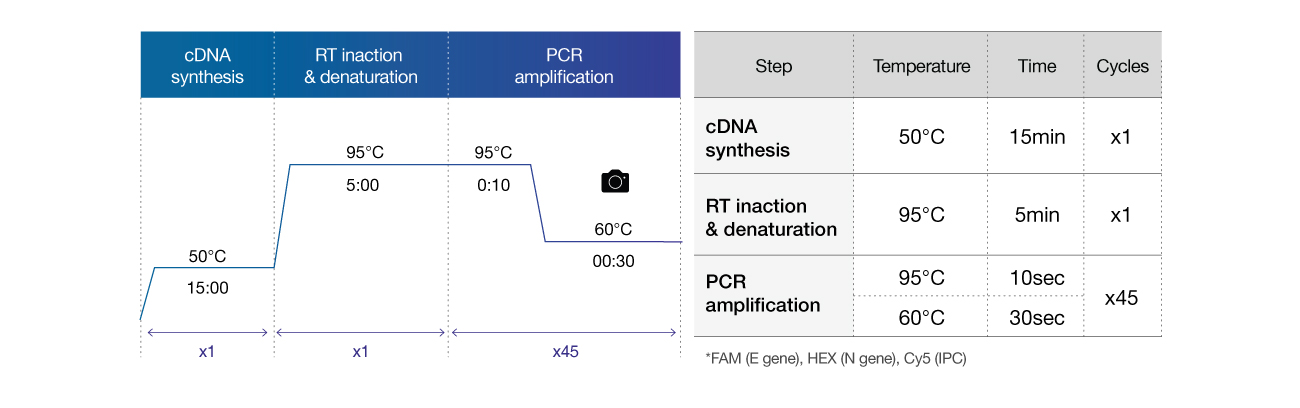
Cycle condition
Reaction conditions of reagents
The En-swer™ COVID-19 RT-PCR Kit can be used with Real-time PCR equipment including 7500 from Applied Biosystems and CFX96 from Bio-Rad. You can also use the equipment that can detect 3 different fluorescence such as FAM, HEX, Cy5, and so on. The response conditions are as follows.
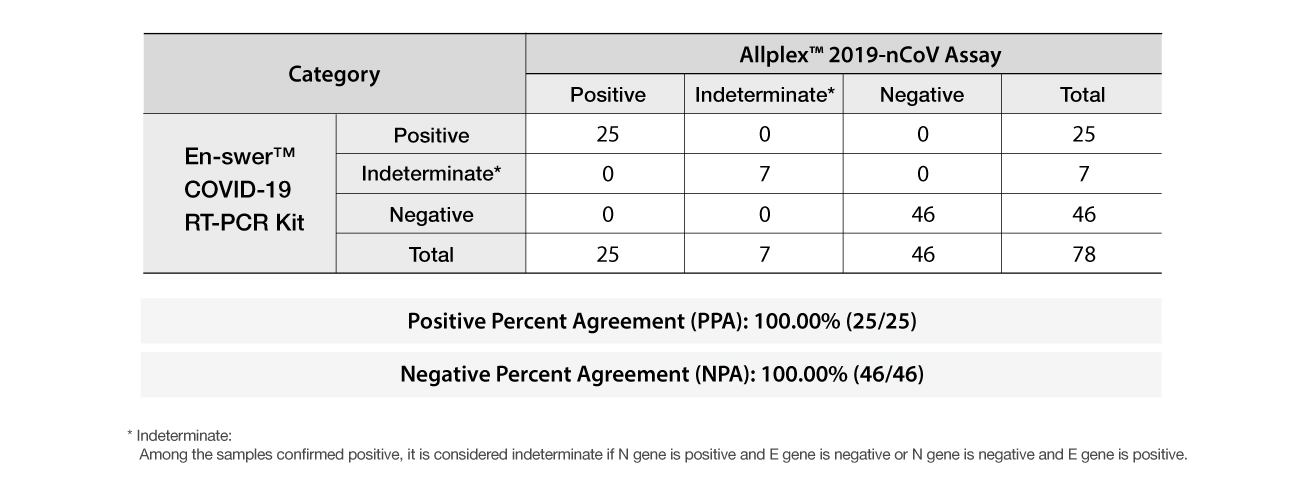
Clinical result
Clinical Performance Evaluation
The PPA (Positive Percent Agreement) and NPA (Negative Percent Agreement) were calculated by comparing the test results of the En-swer™ COVID-19 RT-PCR with that of Allplex™ 2019-nCoV Assay (Korean Emergency Use Authorization approved). The total of 78 samples were compared.
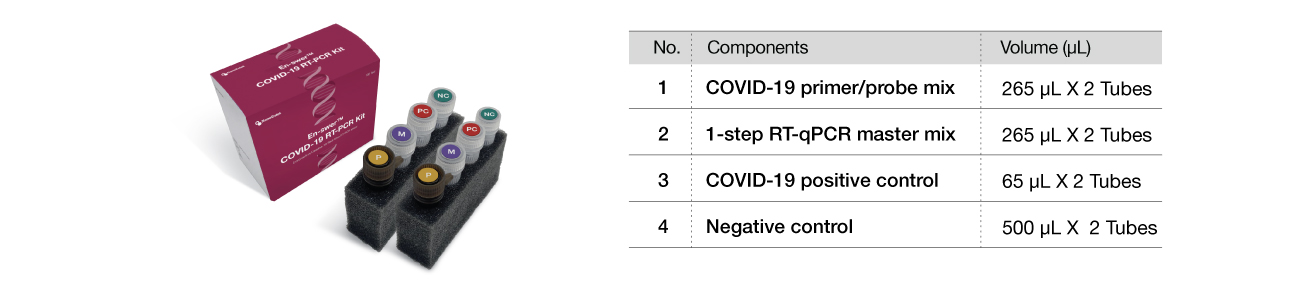
Components
Key components
The following are the components of the En-swer™ COVID-19 RT-PCR Kit.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Chanil Chung
- Address
- Guro-gu, Guro-dong,235-2, Guro-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2000
- No. of Total Employees
- 101-500
- Company introduction
-
- Main Markets
-
 Germany
Germany
 Italy
Italy
 Japan
Japan
 Taiwan
Taiwan
 U. Kingdom
U. Kingdom
- Main Product