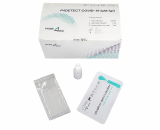
P4DETECT COVID-19 Ag
Rapid chromatographic immunoassay for the qualitative detection of specific antigens to Coronavirus(SARS-CoV-2)
Negotiable Min Order Quantity 10000 Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- OBM,ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- diagnosic kits, rapid test, test kit, covid 19
- Category
- Medical Test Kit
Apply a video call to the Supplier
| Product name | P4DETECT COVID-19 Ag | Certification | CE |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | diagnosic kits , rapid test , test kit , covid 19 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 200000 |
| Supply type | OBM,ODM,OEM | HS code | 3002150000 |
Product Information

P4DETECT COVID-19 Ag
The P4DETECT COVID-19 Ag is quick and easy point-of-care test for the detection of COVID-19 infectio. Oure P4DETECT COVID-19 Ag is rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2.
- Immunochromatography based one-step in vitro diagnostic
- Easy Procedure
- No instruments needed
- Specimen: Nasopharyngeal swab
- Reading Time: 15~20 min
- Storage: 2~30 °C
- - Sensitivity: 96.67%, Specificity: 100.0%
- Test Device
- Extraction Buffer Tube
- Filter Cap
- Sterile Swab
- Instruction for use
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | 10000 | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

Exhibition 1
FTA·AEO Expo-
4


- President
- OH KYUHA
- Address
- 930 415 Heungan daero 14059, Dongan-gu, Anyang-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit
- Year Established
- 2016
- No. of Total Employees
- 1-50
- Company introduction
-
PRIME4DIA is R&D group and manufacturer for in vitro diagnostic products from Republic of Korea. PRIME4DIA is focusing its efforts on Point of Care Testing (POCT) as its core strategic business. Our R&D center and factory located at Anyang city, nearby Seoul, is an up-to-dated manufacturing facility equipped to produce monoclonal antibody, antigen, gold nanoparticle and wide range of rapid tests (semi-finished products).
- Main Markets
-
 Brazil
Brazil
 India
India
 Indonesia
Indonesia
 Kazakhstan
Kazakhstan
 Malaysia
Malaysia
 Peru
Peru
 Russia
Russia
 South Africa
South Africa
- Main Product
Related Products

SELF-STIK SERIES
BIOCREDIT COVID-19 IgG+IgM Duo
BioTracer Troponin I Rapid Test
AFP/PSA/CEA Rapid Test

I-STEM (Y-STEM) PRP KIT



































 South Korea
South Korea