Vstrip Flu A&B Rapid Test
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Taiwan
- Brand name
- Vstrip
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- flu, in vitro diagnostic, rapid test, influenza a&b
- Category
- Medical Test Kit
Apply a video call to the Supplier
Panion&BF Biotech Inc.
- Verified Certificate
-
7

| Product name | Vstrip Flu A&B Rapid Test | Certification | - |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | flu , in vitro diagnostic , rapid test , influenza a&b | Unit Size | - |
| Brand name | Vstrip | Unit Weigh | - |
| origin | Taiwan | Stock | - |
| Supply type | - | HS code | - |
Product Information
Vstrip Flu A&B Rapid Test
INTENDED USE
VstripÒ Flu A&B Rapid Test is a rapid in vitro immunochromatographic assay for the qualitative detection of influenza type A and type B virus from the nasopharyngeal swab of symptomatic patients.
FEATURES
Method: Monoclonal immunochromatographic assay
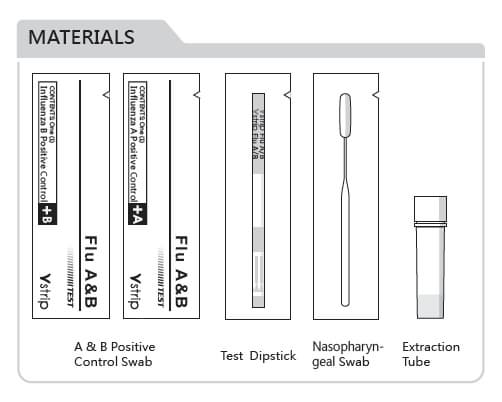
Specimen: Nasopharyngeal swab
Detection Device: Dipstick
Time to Result: 10 minutes
External Quality Control: Positive influenza A and
influenza B control swab
Package: 20 tests/kit
Storage temperature: 15-30℃
CLINICAL PERFORMANCE:
Comparison study was conducted in 132 patients with Flu-like symptoms and compared the results of Vstrip Flu A&B Rapid Test to a FDA-cleared commercial kit.
Type A Type BSensitivity: 94.7 % 91.3 %
Specificity: 97.9 % 100 %
Accuracy: 97.0 % 98.5 %
PPV: 94.7 % 100 %
NPV: 97.9 % 98.2 %
ANALYTICAL SENSITIVITY
Among the 14 influenza A strains (H1N1, H3N2, etc) and 3 influenza B virus(Victoria, Yamagata), the minimum detectable concentration was:
Flu A H1N1 subtype: 1.0 x 104 TCID50/ml
Flu A H3N2 subtype: 5.0 x 103 TCID50/ml
Flu B virus : 1.25 x 105 TCID50/ml
ANALYTICAL SPECIFICITY
The cross reactivity study was evaluated with a total of 15 bacteria strains and 5 viruses strains. None of these tested microorganisms gave a positive result.
CERTIFICATION
ISO 13485
CE Mark
TFDA
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |
- President
- Zong-Ming Jiang
- Address
- 1 F, No.308-8, Sec. 1, Datong Rd., Xizhi Dist., New Taipei City 22146 Taiwan
- Product Category
- Examination & Testing Instrument
- Year Established
- 1976
- No. of Total Employees
- 101-500
- Company introduction
-
- Main Product
Related Products

PRO PRP KIT
BioTracer FOB Rapid Test
Contour Test strips, Accu chek, One Touch, On Call Plus

PREGABLE Ovulation Pregnancy Tests Kit with APP, SmileReader

SELF-STIK SERIES


































 Taiwan
Taiwan

