Se-mi Automated External Defibrillator(AED)
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- RadianQbio
- Payment Terms
- L/C,Others,T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Medical Devices , Medical Consumables
Apply a video call to the Supplier

RADIANQBIO
- Verified Certificate
-
16



| Product name | Se-mi Automated External Defibrillator(AED) | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Medical Consumables |
Ingredients | - |
| Keyword | aed , automated external defibrillator , cardiac arrest , portable defibrillator | Unit Size | 230.0 * 85.0 * 320.0 mm |
| Brand name | RadianQbio | Unit Weigh | 2 kg |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 9018909080 |
Product Information
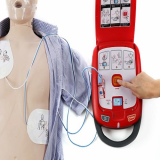
Se-mi Automated External Defibrillator(AED)
HR-501
To save valuable Life
Semi-Automated External Defibrillator for Cardiac arrest
AED helps recover the normal cardiac function upon acute heart disease, such as acute cardiac arrest, by cardiac function analysis and, if necessary, electroshock.
Heart Guardian, as an AED, provides precise analysis of victim’s body and configures the exact amount of shocks, promising the safety and quicker responses.
Easy to use and accessible by all without training
- As soon as uncovering AED, there will be an audio guidance for every phase of operation
- In case where not audio LED indicator guides you for accurate
- From analysis to charging, services are all-automated
- All you need to do is connecting pads at the right place and press a button to work
Compatible with both Adults and kids
- AED pads is compatible among any ages and gender, simply by setting up one button
- Heart Guardian provides the precise amount of shock energy depends on the patient’s size and condition into two levels
Heart Guardian offers data extraction with the embedded memory
- AED offers “defibrillation blackbox” by memorizing the operational history
- Once recorded, data can be transferred out and analyzed by way of Bluetooth enabled AED
│ Product Specification │
|
Model |
HR-501 |
|
Product Type |
Low-output CPR Machine(Folded |
|
ECG Analysis |
8.5sec |
|
Hi-Cap Charging |
8sec |
|
Output |
50J (Child)~150J (Adult) |
|
Data Storage |
100th (ECG storage) |
|
Data Transfer Protocol |
Bluetooth 2.0 |
|
Control Button |
Power, Shock, Analysis |
|
Battery Type |
Li-Mn (12V DC 4.2A) |
|
Shock Frequency |
200, at least |
|
Pad Type |
Electrode Pad(disposable and adhesive) |
|
Dimension |
230(W) x 320(L) x 85(H) |
|
Weight |
1.9kg |
|
Warranty |
5yrs (2yrs for Pads/ 4yrs for batteries) |
|
Paid Repair Service |
10yrs |
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,Others,T/T | Shipping time | Negotiable |

- President
- BEOM KI KIM
- Address
- 1804, Halla-sigma valley,453 gasan digital 2-ro,Geumcheon-gu,seoul,korea
- Product Category
- Medical Analyzer,Medical Devices,Medical Services,Medical Test Kit
- Year Established
- 2005
- No. of Total Employees
- 51-100
- Company introduction
-
[Company Introduction]
RADIANQbio Co., Ltd., a social corporation dedicated to saving lives, was established in October 2005 and has been specialized in R&D, manufacture and sale of sensor, medical devices, measuring equipment, tester, etc., over the last decade.
Particularly, RADIANQbio Co., Ltd., ., has opened up the market for medical and healthcare business in 2014 based on its know-how and expertise amassed in the field of precision measurement and analysis for many years of work, along with its patented technology and technological capability of professional researchers. RADIANQbio Co., Ltd., successfully obtained domestic certification for its automatic defibrillator which culminated about 2 years of R&D in tandem with its localization and is currently proceeding with overseas certification (CE and etc.) to make inroads into the global market.
We will lay the cornerstone for fusion and convergence industrial field as a leader of creative economy while pushing forward ethical management that takes safety and quality as top priority based on the slogan "Life of human is the best value to uphold'
We will make utmost effort to ensure customer satisfaction by fully leveraging our leading technology that sets apart from others.
- Main Markets
-
 Greece
Greece
 Ireland
Ireland
 Italy
Italy
 Lithuania
Lithuania
 Luxembourg
Luxembourg
 Netherland
Netherland
 Poland
Poland
 Portugal
Portugal
 Spain
Spain
 Turkey
Turkey
- Factory Information
-
RADIANQBIO
- Main Product
Related Products

Implant system (SLA surface)

LED Dark Circle Therapy

Modular type ENT workstation_XU5 Visual

Soomplus XG

PRF BOX Implant





































 South Korea
South Korea

_Portable_Defibrillator_2.jpg)
