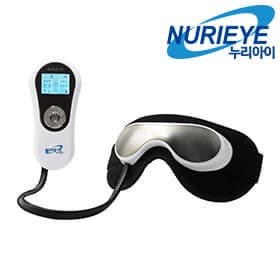
Dry eye treatment medical device nurieye
Dry eye treatment medical device approved by the Ministry of Food and Drug SafetyPublished in British SCI-level ophthalmology journal BJO
$ 100.0 Pieces Min Order Quantity 500 Unit
- Required Quantity
-
- Place of Origin
- Pakistan
- Brand name
- NURIEYE
- Payment Terms
- T/T
- Production method
- OEM
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Beauty Equipment , Body Massagers
Apply a video call to the Supplier
I tech Co.,Ltd.
- Membership
- PRO
- Recent Visit
- Apr 05, 2024
- Country / Year Established
-
 South Korea
/
2014
South Korea
/
2014
- Business type
- Others
- Verified Certificate
-
1

| Product name | Dry eye treatment medical device nurieye | Certification | - |
|---|---|---|---|
| Category |
Beauty Equipment
Body Massagers |
Material | - |
| Keyword | eye massager , medical equipment , dry eye syndrome , intraocular pressure | Unit Size | - |
| Brand name | NURIEYE | Unit Weigh | - |
| origin | Pakistan | Stock | 1000 |
| Supply type | OEM | HS code | - |
Product Information
As a company with a long history of faith and trust, we are a company specializing in dry eye syndrome treatment medical devices that is loved not only domestically but also overseas.
This is a dry eye treatment medical device that has been approved for clinical trials by the government and has been verified for safety and effectiveness through clinical trials on 144 dry eye patients at two government-designated clinical trial centers at two university general hospitals.
Among the medical vibrator items approved as medical devices for treating dry eye syndrome, as of March 2018, the only product that has been clinically tested and approved through clinical data review at a designated clinical testing institution in accordance with the Ministry of Food and Drug Safety Notice is the Nuriye-5800 product.
The skin and muscles around the eyes harden and the circulation of nerves, blood vessels, and tears is disrupted, resulting in a decrease in the amount or change in the quality of tears, resulting in dry eyes. NURIEYE gently relaxes the skin muscles around the eyes with vibration and air pressure massage, thereby facilitating circulation of nerves, blood vessels, and tears. It also uses warm heat massage to remove foreign substances buried or accumulated in the eye and hardened fat in the meibomian glands inside the eyelids. It dissolves secretions and aids tear circulation to treat dry eyes.
Nurieye, a medical device for treating dry eye syndrome, provides 15 types of fine vibration massage to relieve stiff muscles around the eyes + low-temperature/high-temperature heat massage to melt fatty oil accumulated in the eyelids + expel the melted fatty oil through heat so that it does not harden in place. It consists of a 5-stage pneumatic massage that promotes smooth circulation of natural tears to treat dry eye syndrome.
It is a dry eye treatment medical device that has been proven safe and effective through clinical trials by the Ministry of Food and Drug Safety, and the effectiveness of NURIEYE-5800 in treating dry eye syndrome was published and published in the British Journal of Ophthalmology, a British ophthalmology medical journal. In addition, the evaluation of new medical technology has been recognized and the Ministry of Health and Welfare has announced that it is not covered by health insurance, and NURIEYE dry eye treatment medical devices are being treated at eye hospitals across the country.
1) Air pressure massage: 5 levels of soft air pressure massage around the eyes
2) Thermal massage: Warm thermal compress (low temperature: 30 to 45 degrees, high temperature: 45 to 55 degrees)
3) Vibration massage: 15 massage modes with fine vibration technology
4) Convenient LCD controller (Korean/English): Mode. Time setting: automatic/manual
B2B Trade
| Price (FOB) | 100.0 USD | transportation | Air Transportation |
|---|---|---|---|
| MOQ | 500 | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |
I tech Co.,Ltd.
- Country / Year Established
-
 South Korea
/
2014
South Korea
/
2014
- Membership
- PRO
- Recent Visit
- Apr 05, 2024
- Business type
- Others
-
1

- President
- CHANGON, KIM
- Product Category
- Medical Devices
- Year Established
- 2014
- Main Markets
-
 Australia
Australia
 Hong Kong(China)
Hong Kong(China)
 Kazakhstan
Kazakhstan
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Main Product







































_thread_(Cog,_Mold,_Screw,_multi)_2.jpg)

